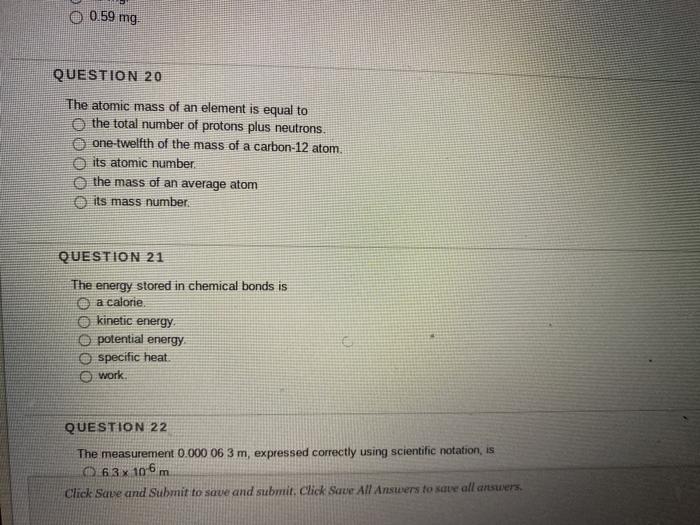
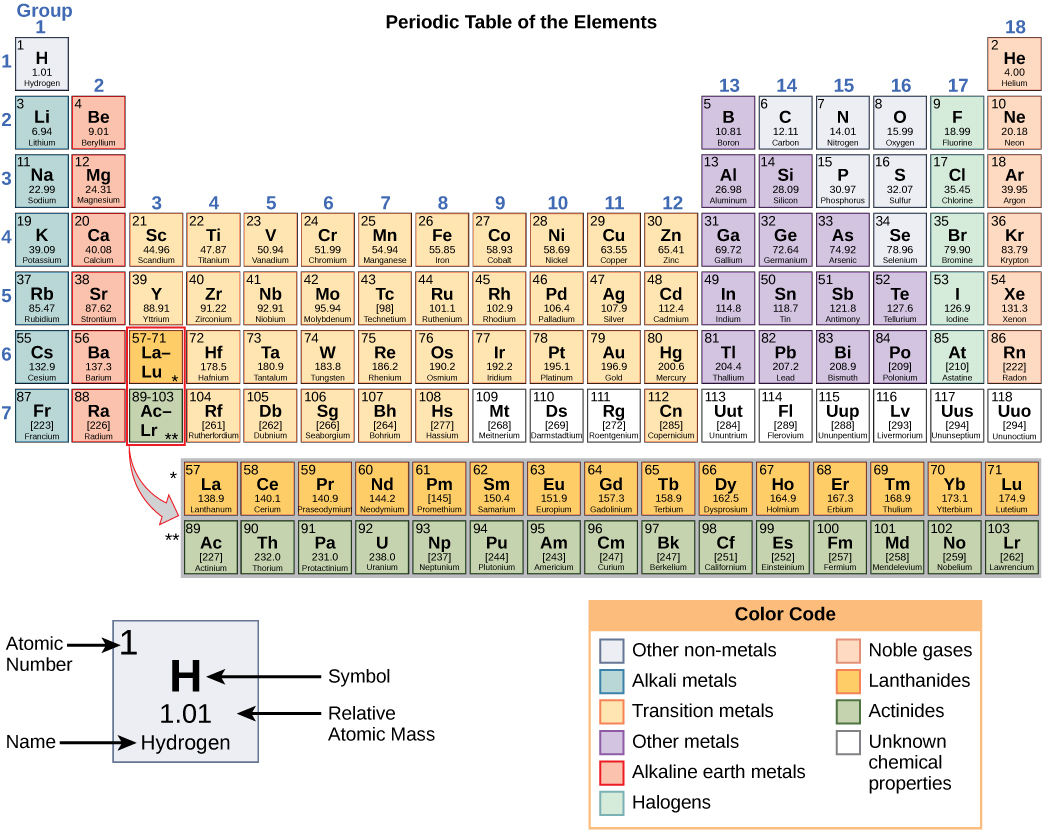
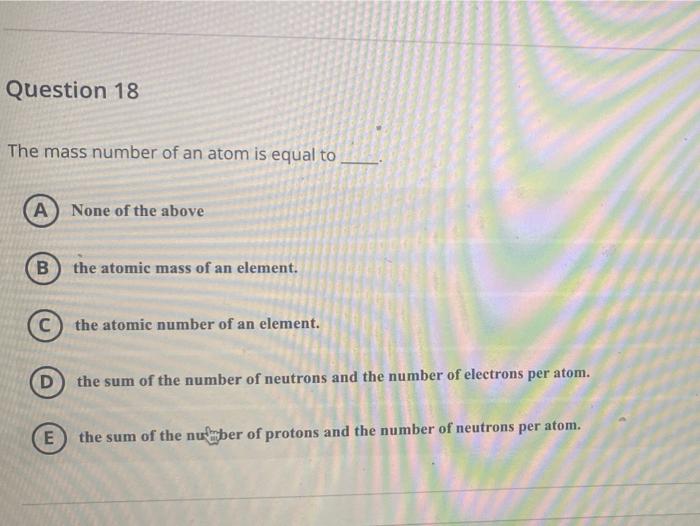
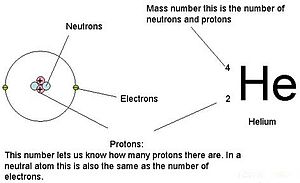
1 Atomic Mass The atomic mass of an element is listed below the symbol of each element on the periodic table. gives the mass of an “average” atom of each. - ppt download

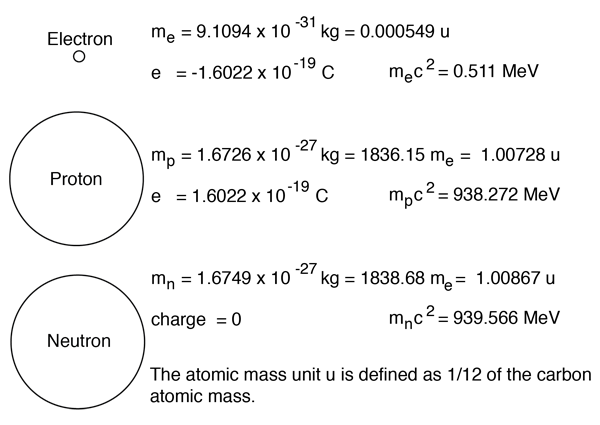
Therefore: There are 3 subatomic particles: protons, neutrons and electrons. These are measured in “ atomic mass units ” ( amu ) as their mass is so small. - ppt download
What's the math behind the similar numerical values of molar mass and relative molecular/atomic mass? - Quora

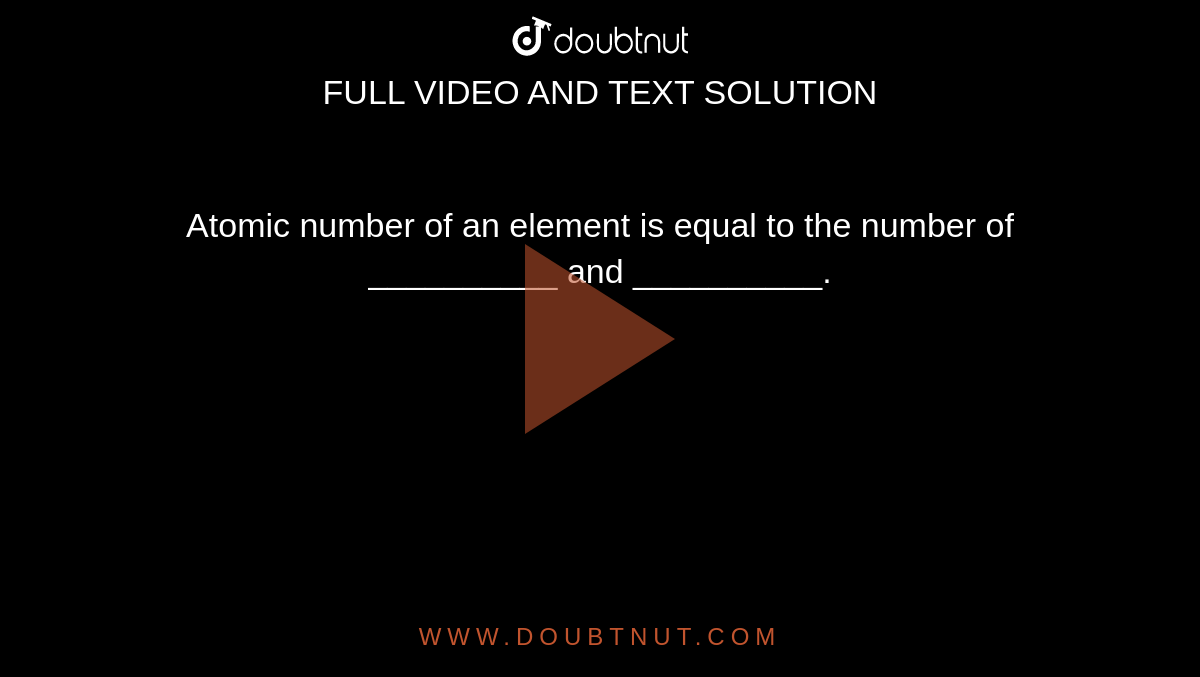
SOLVED: Atomic Number of an element is equal to:-(a) Number of Protons (b) Number of electrons(c) Number of neutrons (d) Both a) and b)
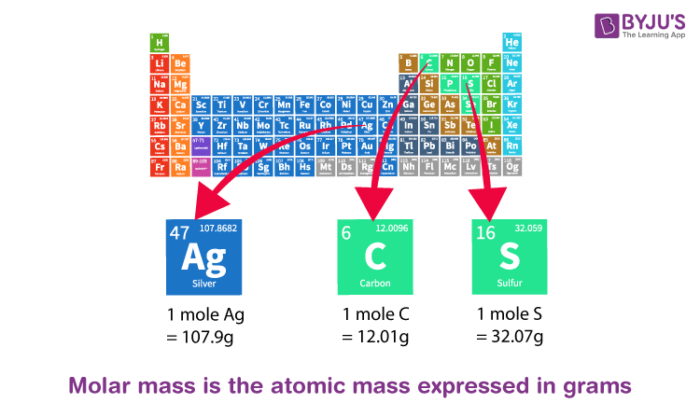
What is Atomic Mass? - List of Elements Sorted by Atomic Mass of Iron, Sulphur, Potassium, Chlorine Etc

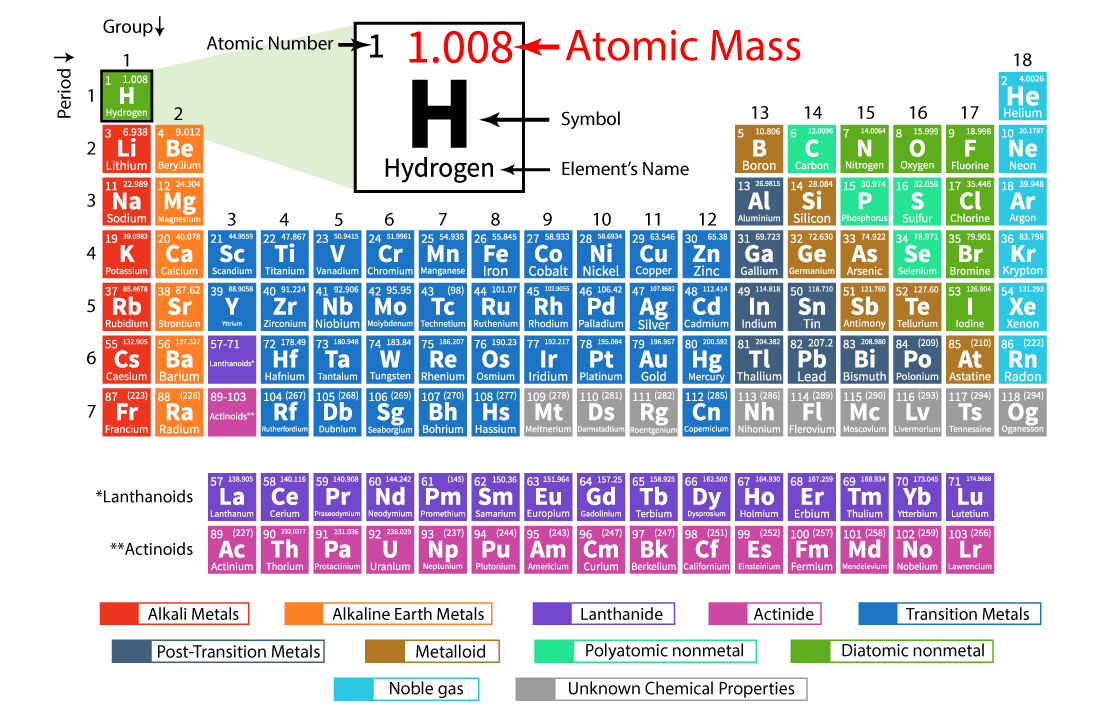
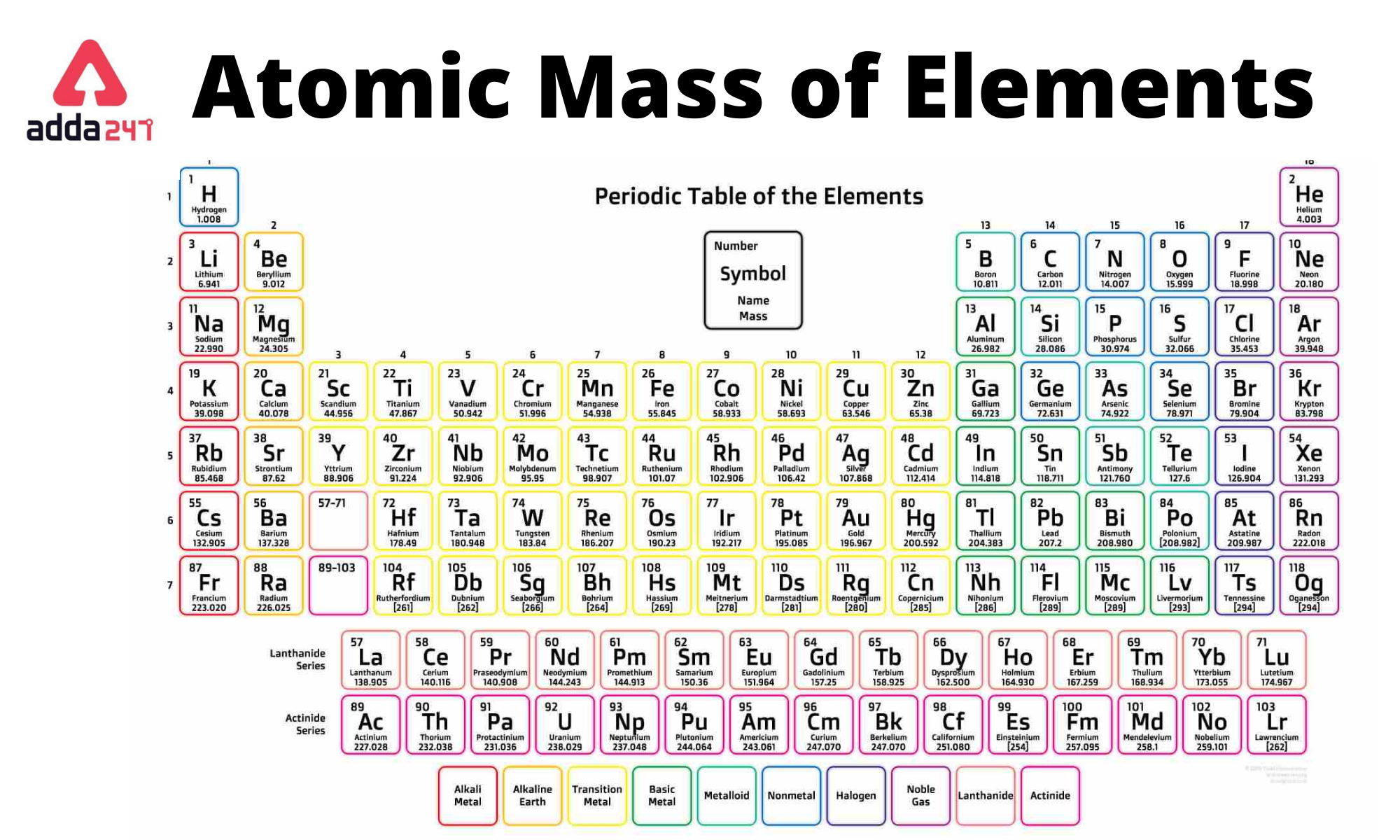
:max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)